Experience
Venofer® SELECTED CLINICAL STUDIES
Back to clinical studiesThis study led to the FDA approval of Venofer. Always refer to the approved Full Prescribing Information and Important Safety Information below.
Comparison of the safety and efficacy of 3 iron sucrose iron maintenance regimens in children, adolescents and young adults with CKD: a randomized controlled trial. A multicenter trial of IV iron sucrose injection (Venofer; Luitpold Pharmaceuticals, Inc.) in children, adolescents and young adults with CKD Maintenance dosing with Venofer demonstrates safety and efficacy in chronic kidney disease (CKD) patients ≥ 2 years receiving erythropoiesis-stimulating agents (ESAs).1
Significant Percentage of Venofer Patients Maintained 10.5-14.0 g/dL Hemoglobin Range Over 12 Weeks Across 3 Doses2
Study Design/Methods1,2
Study F was a randomized, open-label, dose-ranging study for iron maintenance treatment in pediatric patients with dialysis-dependent or non-dialysis-dependent (NDD) including PDD chronic kidney disease (CKD) on stable erythropoietin therapy. The study randomized patients to 1 of 3 doses of Venofer (0.5 mg/kg, 1 mg/kg or 2 mg/kg). The mean age was 13 years (range 2 to 21 years). Over 70% of patients were over 12 years of age in all 3 groups. There were 84 males and 61 females. About 60% of patients underwent hemodialysis and 25% underwent peritoneal dialysis in all 3 dose groups. At baseline, the mean hemoglobin for each of the 3 groups was 12.22 for 0.5 mg/kg, 12.19 for 1 mg/kg, and 12.03 for 2 mg/kg. The mean transferrin saturation (TSAT) for each of the 3 groups was 33.0%, 33.2%, and 31.9%, respectively. The mean ferritin for each of the 3 groups was 262 ng/mL, 308 ng/mL, and 326 ng/mL, respectively. Patients with hemodialysis dependent-chronic kidney disease (HDD-CKD) received Venofer once every other week for 6 doses. Patients with peritoneal dialysis dependent-chronic kidney disease (PDD-CKD) or NDD-CKD received Venofer once every 4 weeks for 3 doses.
Safety was the primary end point. Safety was determined through number of adverse events, clinical laboratory evaluations, vital signs and physical examinations. The main clinical end point was a composite of hemoglobin level ≥10.5-14.0 g/dL, inclusive; transferrin saturation ≥20%-50%, inclusive; and stable ESA dosing (±25% of baseline dose). A dose-response relationship was not demonstrated. There was no difference in reported adverse events between the lower and higher doses. The study drug was administered undiluted over 5 minutes by IV push or diluted in 25 mL of 0.9% sodium chloride solution and administered over 5 to 60 minutes; the duration of IV push/infusion was determined by local institutional protocol.
Although site investigators could not alter IV iron doses, they were free to increase or decrease ESA dosing for safety concerns. stable ESA dosing rates were 93% to 100%.1
None of the patients studied experienced a drug-related anaphylactic reaction while on iron sucrose. No differences were noted between regimens in reported adverse effects, which were all minor.1
Common Adverse Events1
System Organ Class
≥ 1 treatment-emergent adverse event
Gastrointestinal
Infections and infestations
Nervous system disorders
Respiratory, thoracic, and mediastinal disorders
System Organ Class
Iron Sucrose Group
Iron Sucrose Group
0.5 mg/kg (n=47)
1 mg/kg (n=47)
2 mg/kg (n=47)
≥ 1 treatment-emergent adverse event
27 (57)
25 (53)
26 (55)
Gastrointestinal
6 (13)
9 (19)
6 (13)
Infections and infestations
10 (21)
9 (19)
7 (15)
Nervous system disorders
5 (11)
5 (11)
7 (15)
Respiratory, thoracic, and mediastinal disorders
3 (6)
1 (2)
3 (6)
Percentage of venofer patients that maintained 10.5-14.0 g/dL Hemoglobin Over 12 weeks1,2

Individual end points1
End Point
Iron Sucrose Group
Clinical successa
0.5 mg/kg (n=46)
1.0 mg/kg (n=45)
2.0 mg/kg (n=40)
All Patients
12/46 (26 [13-39])
10/45 (22 [10-34])
12/40 (30 [16-44])
PD/NDD-CKDb
4/17 (24 [3-44])
1/16 (6 [0-18])
7/16 (44 [19-68])
HD
8/29 (28 [11-44])
9/29 (31 [14-48])
5/24 (21 [5-37])
Hb 10.5-14.0 g/dLC
All Patients
27/46 (59 [44-73])
21/45 (47 [32-61])
18/40 (45 [30-60])
PD/NDD-CKD
10/17 (59 [35-82])
5/16 (31 [9-54])
8/16 (50 [26-75])
HD
17/29 (59 [41-77])
16/29 (55 [37-73])
10/24 (42 [22-61])
TSAT 20%-50%C
All Patients
15/46 (33 [19-46])
18/45 (40 [26-54])
20/40 (50 [35-65])
PD/NDD-CKD
4/17 (24 [3-44])
6/16 (38 [14-61])
9/16 (56 [32-81])
HD
11/29 (38 [20-56])
12/29 (41 [24-59])
11/24 (46 [26-66])
Stable ESA dosing
All Patients
46/46 (100 [NC])
45/45 (100 [NC])
39/40 (98 [93-100])
PD/NDD-CKD
17/17 (100 [NC])
16/16 (100 [NC])
16/16 (100 [NC])
HD
29/29 (100 [NC])
29/29 (100 [NC])
23/24 (96 [88-100])
Hb ≥ 10.5 g/dLd
30/46 (65 [51-79])
25/45 (56 [41-70])
22/40 (55 [40-70])
TSAT ≥ 20%d
30/46 (65 [51-79])
32/45 (71 [58-84])
32/40 (80 [68-92])
Hb ≥ 10.5 g/dL and TSAT ≥ 20%d,e
16/46 (35 [21-49])
11/45 (24 [12-37])
17/40 (43 [27-58])
NOTE: Values are presented as n/N (percentage [95%Cl]). The 95% CI is based on normal approximation to binomial distribution.
Abbreviations: CI, confidence interval; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; HD, hemodialysis; NC, not calculated; NDD-CKD, non-dialysis dependent
chronic kidney disease; PD, peritoneal dialysis; TSAT, transferrin saturation.
aClinical success indicated Hb level of 10.5-14.0 g/dL, inclusive; TSAT of 20%-50%, inclusive; and stable ESA dosing defined as no more than a 25% increase or 25% decrease from baseline ESA dose during the study period.
bP = 0.04 for the 2.0-mg/kg vs 1.0-mg/kg dosing arm.
cInclusive.
dAll patients.
ePost hoc combined end point is Hb level ≥ 10.5 g/dL and TSAT ≥ 20% during the entire study period.
Based on current data, This study provided evidence that the lower 0.5 mg/kg dosing regimen be used with regular monitoring and adjustment of iron and ESA therapy. This study led to the FDA approval of VENOFER AT 0.5 mg/kg FOR PEDIATRIC PATIENTS ≥ 2 YEARS AND OLDER FOR IRON MAINTENANCE THERAPY. Always refer to the approved Full Prescribing Information.1,2
Target Success Rate for Hemoglobin ≥ 10.5 g/dL1*

Dosage 0.5 mg/kg

Dosage 1.0 mg/kg

Dosage 2.0 mg/kg
*Removing the upper limit of hemoglobin (14 g/dL) and TSAT (50%).
Change in mean hemoglobin level among the 3 dosing groups during the course of the study1

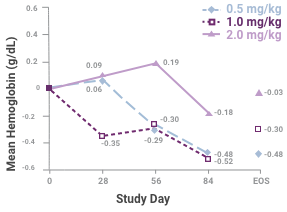
Samples sizes at each time are 45, 45, 41 and 34 for the 0.5-mg/kg group; 46, 41, 35 and 35 for the 1.0-mg/kg group; and 43, 37, 34 and 33 for the 2.0-mg/kg group. Abbreviation: EOS, end of study. This study led to the FDA approval for Venofer. Always refer to the approved Full Prescribing Information.
Change in mean serum ferritin level among the 3 dosing groups during the course of the study1
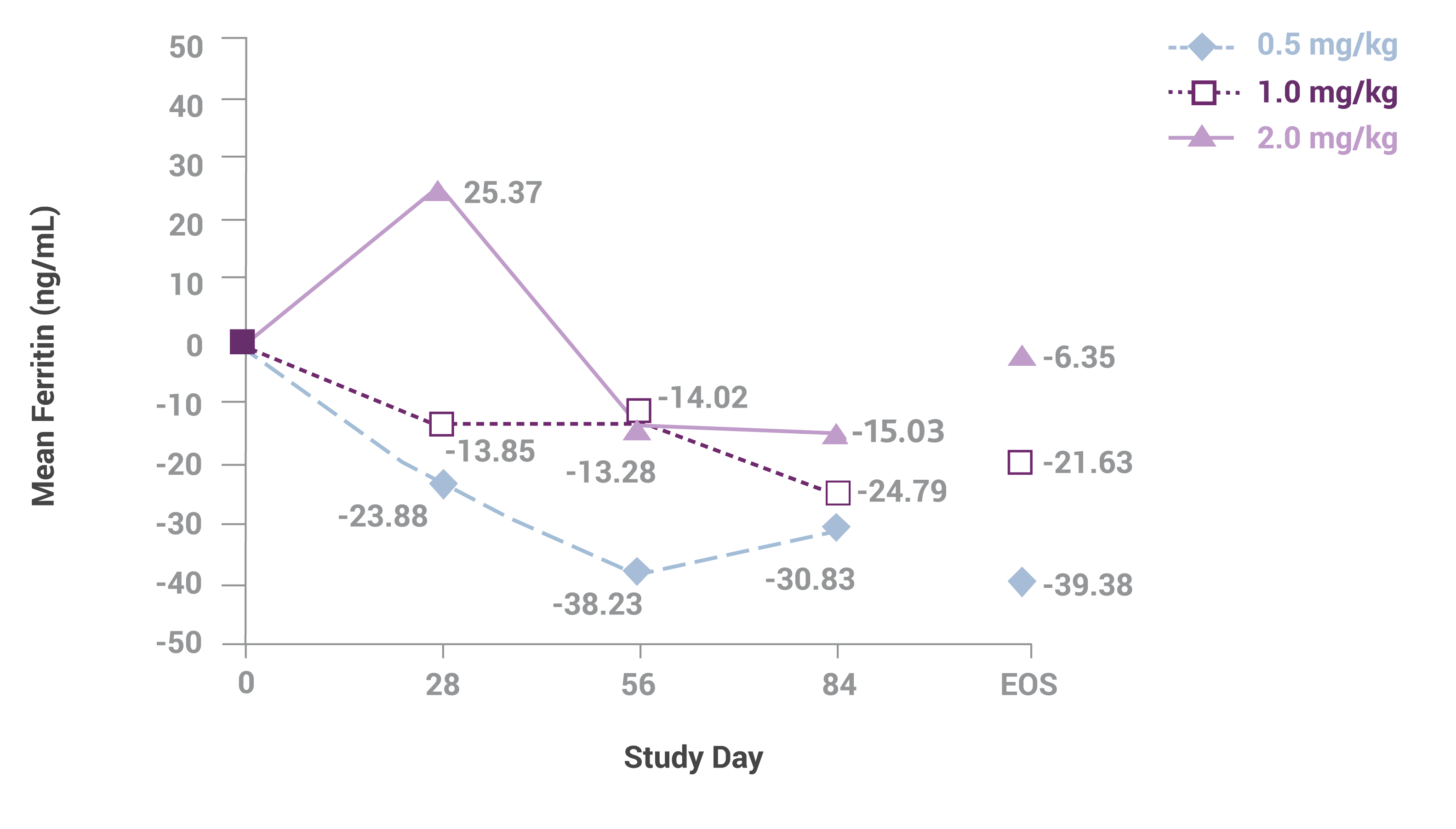
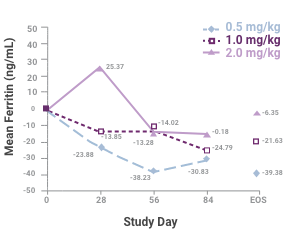
Sample sizes at each time are 45, 45, 41 and 34 for the 0.5-mg/kg group; 46, 41, 35 and 35 for the 1.0-mg/kg group; and 43, 37, 34 and 33 for the 2.0-mg/kg group. Abbreviation: EOS, end of study. This study led to the FDA approval for Venofer in pediatric patients. Always refer to the approved Full Prescribing Information.
Change in mean transferrin saturation (TSAT) among the 3 dosing groups during the course of the study1

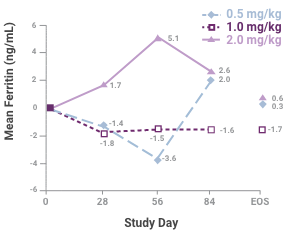
Samples sizes at each time are 45, 45, 41 and 34 for the 0.5-mg/kg group; 46, 41, 35, and 35 for the 1.0-mg/kg group; and 43, 37, 34 and 33 for the 2.0-mg/kg group. Abbreviation: EOS, end of study. This study led to the FDA approval for Venofer. Always refer to the approved Full Prescribing Information
A single dose of Venofer given every other week for children, adolescents and young adults receiving HD and monthly for such patients on PD therapy or with NDD-CKD maintains the target hemoglobin with equivalent safety over a limited follow-up.1
NOTE: These data are not drawn from the pivotal trials that led to the FDA approval of Venofer. These data are provided by a post-market study that supports the Venofer approved indication.
Venofer has established safety profile as iron maintenance therapy in pediatric patients (over 2 years old)1,2
This study established a safety profile for Venofer as an iron maintenance treatment in pediatric patients with dialysis-dependent or non-dialysis-dependent CKD on stable erythropoietin therapy. The study randomized patients to 1 of 3 doses of Venofer (0.5 mg/kg, 1.0 mg/kg or 2.0 mg/kg). The mean age was 13 years (range 2 to 20 years). None of the patients studied experienced a drug-related anaphylactic reaction while on iron sucrose. Venofer has not been studied in patients younger than 2 years old.
During the study, at least one adverse event was experienced by 27 of 47 (57.4%) patients in the 0.5-mg/kg group, 25 of 47 (53.2%) in the 1.0-mg/kg group, and 26 of 47 (55.3%) in the 2.0-mg/kg group. The most common (≥5% of patients) adverse event in the 0.5-mg/kg group was respiratory tract infection (8.5%). The most common adverse events in the 1.0-mg/kg group were headache (8.5%), vomiting (6.4%) and peritonitis (6.4%). The most common adverse events in the 2.0-mg/kg group were increased blood pressure and cough (6.4% each). Only one patient (in the 2.0-mg/kg group) did not complete the study because of an adverse event, which was not considered to be related to the study drug.
IV iron sucrose at a dose of 0.5 mg/kg at the intervals prescribed is noninferior to higher doses in maintaining hemoglobin levels of ≥10.5 g/dL in children, adolescents and young adults receiving ESA therapy.
According to the labeling, Feraheme and Injectafer are not indicated for pediatric patients.3,4 Ferrlecit is indicated in adults and children 6 years and older.5
Venofer is the most studied IV iron drug for children, adolescents and young adults2,3
This study was the largest prospective evaluation to date of IV iron therapy in children, adolescents and young adults with CKD.
REFERENCES
1. Venofer® [package insert]. Shirley, NY: American Regent, Inc.; 2019.
2. Goldstein SL, Morris D, Warady, BA. Comparison of the safety and efficacy of 3 iron sucrose Iron maintenance regimens in children, adolescents, and young adults with CKD: a randomized controlled trial. Am J Kidney Dis. 2013;61(4):588-597.
3. Feraheme® [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2018.
4. Injectafer® [package insert]. Shirley, NY: American Regent, Inc.; 2018.
5. Ferrlecit® [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2011.
6. U.S. National Library of Medicine. ClinicalTrials.gov. Results for "iron maintenance pediatric" and "IV iron pediatric." https://clinicaltrials.gov. Accessed February 2020.
STUDY REFERENCES
1. Goldstein SL, Morris D, Warady BA. Comparison of the safety and efficacy of 3 iron sucrose Iron maintenance regimens in children, adolescents, and young adults with CKD: a randomized controlled trial. Am J Kidney Dis. 2013;61(4):588-597.
2. Venofer® [package insert]. Shirley, NY: American Regent, Inc.; 2019.

