Experience
Venofer® SELECTED CLINICAL STUDIES
Back to clinical studiesThis study led to the FDA approval of Venofer. Always refer to the approved Full Prescribing Information and Important Safety Information below.
VENOFER VERSUS ORAL FERROUS SULFATE
Venofer is a more efficacious option for iron replacement therapy in NDD-CKD patients compared to oral ferrous sulfate1
Patients given Venofer demonstrated a superior effect on hemoglobin compared to those given oral iron, with statistically significant effects observed in as little as 14 days.
Study design
This study was a randomized, open-label, multicenter, active-controlled study of the safety and efficacy of oral iron versus Venofer in patients with NDD-CKD with or without erythropoietin therapy. Erythropoietin therapy (if any was given) was stable for 8 weeks prior to randomization. In the study, 188 patients with NDD-CKD, hemoglobin ≤11.0 g/dL, transferrin saturation ≤25%, and ferritin ≤300 ng/mL were randomized to receive either oral iron (325 mg ferrous sulfate 3 times daily for 56 days) or Venofer (either 200 mg over 2 to 5 minutes, administered 5 times within 14 days, or two 500 mg infusions on Day 1 and Day 14, administered over 3.5 to 4 hours). The mean ages in the intention to treat analysis were 62.3 years for the 79 treated patients in the Venofer group and 63.9 years for the 82 patients in the oral iron group. The proportion of patients achieving an Hb increase ≥1 g/dL was the primary endpoint.
Hemoglobin response in anemic CKD patients after IV iron compared to oral iron therapy

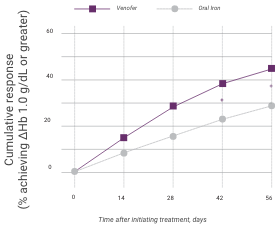
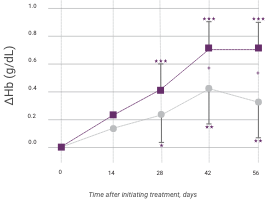
Venofer (IV iron), n=79; ferrous sulfate (oral iron), n=81. Proportion of patients achieving primary endpoint (ΔHb ≥1.0 g/dL), left panel; and actual ΔHb, right panel (mean ±95% CI of mean). Between treatment groups, significant differences were present by day 42 (P<0.05). Within each group, significant differences from baseline are shown (*P<0.05, **P<0.01, ***P<0.001).
In addition to hemoglobin, Venofer also shows significantly greater effects on ferritin and transferrin saturation (TSAT) levels compared to oral iron.
Change in iron indicators in anemic CKD patients after Venofer compared to oral iron therapy (mean ±95% CI of mean)

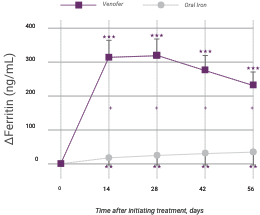
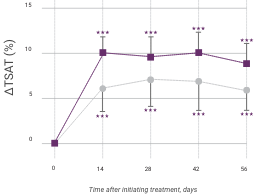
Venofer (IV iron), n=79; ferrous sulfate (oral iron), n=81. Between-group differences were significant for ferritin (P<0.0001) and TSAT (P=0.0411). Within each group, significant differences from baseline are shown (**P<0.01, ***P<0.001).
Venofer helps preserve glomerular filtration rate (GFR) compared to oral ferrous sulfate
More pronounced decreases in GFR were observed among patients in the oral iron group compared to those in the Venofer group (P=0.01).
Study group
Change in GFR from baseline during the study*
P-value
Venofer
-1.45 mL/min/1.73 m2
95% CI -0.2 to -2.67; P=0.0208
Oral iron
-4.40
95% CI -2.5 to -6.29; P<0.0001
*GFR predicted by the Modification of Diet in Renal Disease (MDRD) formula.2
ADHERENCE IS CRITICAL IN IRON REPLACEMENT THERAPY
Venofer was associated with higher adherence rates compared to oral ferrous sulfate.

Venofer was associated with ~20% increased adherence rates compared to oral ferrous sulfate.
Oral ferrous sulfate demonstrated several gastrointestinal (GI) adverse events (AEs), including constipation, nausea, vomiting, and diarrhea. Venofer 500 mg was generally well tolerated, demonstrating a lower AE rate compared to oral ferrous sulfate.
Most frequently reported adverse events (AEs) by treatment arm
Venofer group
Oral iron group
AE
200 mg x 5
(N=61)
500 mg x 2
(N=30)
325 mg TID x 56
(N=91)
Gastrointestinal
7 (11.5%)
1 (3.3%)
16 (17.6%)
Constipation
1 (1.6%)
8 (8.8%)
Diarrhea
3 (3.3%)
Nausea/vomiting
2 (3.3%)
5 (5.5%)
Dyspepsia
1 (1.6%)
1 (1.1%)
Dysgeusia
4 (6.6%)
1 (3.3%)
Headache
2 (3.3%)
Myalgia
2 (3.3%)
1 (3.3%)
Hypotension
2 (6.7%)
IV iron group
ADE
200 mg x 5
(N = 61)
500 mg x 2
(N = 30)
Gastrointestinal
7 (11.5%)
1 (3.3%)
Constipation
1 (1.6%)
Diarrhea
Nausea/vomiting
2 (3.3%)
Dyspepsia
1 (1.6%)
Dysgeusia
4 (6.6%)
1 (3.3%)
Headache
2 (3.3%)
Myalgia
2 (3.3%)
1 (3.3%)
Hypotension
2 (6.7%)
Oral iron group
ADE
325 mg tid x 56
(N = 91)
Gastrointestinal
16 (17.6%)
Constipation
8 (8.8%)
Diarrhea
3 (3.3%)
Nausea/vomiting
5 (5.5%)
Dyspepsia
1 (1.1%)
Dysgeusia
Headache
Myalgia
Hypotension
One serious adverse event (hypotension) was observed in 2 patients with 500 mg Venofer.
Venofer is more efficacious than oral iron
This study adds to the literature that demonstrates that IV iron replacement is a better alternative to oral ferrous sulfate across multiple indicators.1-3 There is consistent evidence that supports earlier, significant changes in hemoglobin, ferritin and TSAT with IV iron in CKD patients requiring iron replacement therapy.1-3 In this study, patients achieved statistically increased hemoglobin in as little as 14 days with Venofer.1
Venofer has demonstrated a better tolerability profile compared to oral iron products
Since oral iron products are absorbed in the GI tract, they are often associated with GI adverse events.1-3 These can be severe and may lower patient adherence.1,4,5 This study supported this by showing a >20% reduced adherence rate compared to Venofer.1
Venofer is an effective and well-tolerated option for iron replacement for individuals with NDD-CKD. Compared to oral iron therapy, Venofer demonstrated a more rapid replacement of iron which positively impacted several iron indices, including hemoglobin, and patient adherence.
STUDY REFERENCES
1. Van Wyck DB, Roppolo M, Martinez CO, et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68(6):2846-2856.
2. Klahr S. Role of dietary protein and blood pressure in the progression of renal disease. Kidney Int. 1996;49:1783-1786.
3. Shepshelovich D, Rozen-Zvi B, Avni T, et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68(5):677-690.
4. O'Lone EL, Hodson EM, Nistor I, et al. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2019;2:CD007857.
5. Stein J, Connor S, Virgin G, Ong DE, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22(35):7908-7925.

