Experience
Venofer® SELECTED CLINICAL STUDIES
Back to clinical studiesThis study led to the FDA approval of Venofer. Always refer to the approved Full Prescribing Information and Important Safety Information below.
This was an open-label, single-arm, prospective, multicenter study to assess the efficacy and safety of IV iron sucrose injection for the treatment of iron deficiency anemia in hemodialysis patients undergoing epoetin therapy for dialysis-associated anemia.1,2
Venofer was evaluated in hemodialysis-dependent chronic kidney disease (HDD-CKD) patients taking erythropoietin.1
Venofer demonstrated a significant increase in hemoglobin, TSAT and ferritin compared to historical controls WITHIN 5 WEEKS AFTER TREATMENT PERIOD1,2
Venofer (iron sucrose) injection, USP is an effective and well-tolerated option for iron replacement for individuals with dialysis-associated anemia. Patients showed a significant increase in hemoglobin (Hb) levels after just 3 doses of Venofer and maintained this increase for at least 5 weeks after the last dose (10th dose).2
Study Design/Methods1,2
Study A was an open-label, single-arm, prospective, multicenter study to assess the efficacy and safety of IV iron sucrose injection for the treatment of iron deficiency anemia in hemodialysis patients undergoing epoetin therapy for dialysis-associated anemia. Of the 101 patients studied, 77 were treated with Venofer (iron sucrose) injection, USP, and 24 were in the historical control group. Eligibility criteria for Venofer treatment included patients undergoing chronic hemodialysis and receiving erythropoietin, with a hemoglobin level between 8.0 and 11.0 g/dL, transferrin saturation <20% and serum ferritin <300 ng/mL. The mean age of the patients was 65 years, with ages ranging from 31 to 85 years. Of the 77 patients, 44 (57%) were male and 33 (43%) were female.
Trials included patients with iron dextran sensitivity, other drug allergy or concurrent angiotensin-converting enzyme inhibitor use. Patients were administered iron sucrose injection, 100 mg, undiluted, by IV push over 5 minutes. Injections were initiated within the first 30 minutes after starting dialysis. All concomitant medications were recorded. Oral iron agents were precluded by protocol. Epoetin dose adjustments were precluded by protocol, with exceptions made at the request of the investigator when deemed in the best interest of the patient. Patients administered angiotensin-converting enzyme (ACE) inhibitors at enrollment continued these medications throughout the observation and treatment periods. The historical control population consisted of 24 patients with similar ferritin levels as patients treated with Venofer, who were off intravenous iron for at least 2 weeks and who had received erythropoietin therapy, with hematocrit averaging 31 to 36 for at least 2 months prior to study entry. The mean age of patients in the historical control group was 56 years, with an age range of 29 to 80 years. Patient age and serum ferritin level were similar between treatment and historical control patients.
Changes From Baseline in Hemoglobin and Hematocrit1
Efficacy
Parameters
Hemoglobin (g/dL)
Hematocrit (%)
Efficacy Parameters
End of Treatment
End of Treatment
Venofer
(n=69)
Historical Control
(n=18)
Hemoglobin (g/dL)
1.0 ± 0.12**
0.0 ± 0.21
Hematocrit (%)
3.1 ± 0.37**
-0.3 ± 0.65
Efficacy Parameters
2-Week Follow-Up
2-Week Follow-Up
Venofer
(n=73)
Historical Control
(n=18)
Hemoglobin (g/dL)
1.3 ± 0.14**
-0.6 ± 0.24
Hematocrit (%)
3.6 ± 0.44**
-1.2 ± 0.76
Efficacy Parameters
5-Week Follow-Up
5-Week Follow-Up
Venofer
(n=71)
Historical Control
(n=15)
Hemoglobin (g/dL)
1.2 ± 0.17*
-0.1 ± 0.23
Hematocrit (%)
3.3 ± 0.54
0.2 ± 0.86
**P < 0.01 and *P < 0.05 compared to historical control from ANCOVA analysis, with baseline hemoglobin, serum ferritin, and erythropoietin dose as covariates.
Serum ferritin increased at endpoint of study from baseline in the Venofer-treated population (165.3 ± 24.2 ng/mL) compared to the historical control population (-27.6 ± 9.5 ng/mL). Transferrin saturation increased at endpoint of study from baseline in the Venofer-treated population (8.8% ± 1.6%) compared to this historical control population (-5.1% ± 4.3%).
Venofer demonstrated significant increases in mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), iron, TRANSFERRIN SATURATION (TSAT), and ferritin levels, while total iron binding capacity (TIBC) decreased significantly.2
The change from day 1 baseline results in each patient were calculated, the one-sample 95% confidence interval (CI) around the mean result for all patients was computed on each day, and the change from baseline was concluded to be statistically significant at P < 0.05 if the resulting CI did not include the value zero.





Anemia and iron indices before (day 1), during (days 8 to 22) and after (days 24 to 57) iron sucrose administration for iron deficiency. Arrows indicate timing of each of ten 100-mg doses of iron sucrose. Hb levels were significantly greater on day 8 than at baseline and remained greater than baseline throughout the 57-day study period. Epoetin doses were to be held constant by protocol and did not change significantly.
*The computed 95% CIs around the mean change from baseline do not include the value zero.
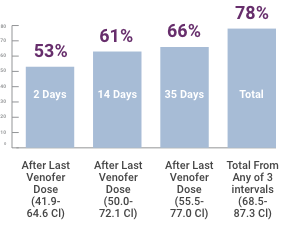
78% of patients attained the target Hb level (≥ 11.0 g/dL) after treatment2
Patients Attaining Hb Level ≥ 11.0 g/dL After Ten 100-mg Doses of Venofer2

This study demonstrated efficacy outcomes of iron sucrose for iron deficiency in patients with dialysis-associated anemia. These include2:
- A significant increase in Hb level was observed at day 8 after administration of 300 mg of iron sucrose. Hb levels peaked at day 36, 2 weeks after administration of the last dose of iron sucrose. A significant increase was also observed at day 57, 5 weeks after the last dose (total dose of 1000 mg)
- More than 70% of the patients showing iron deficiency achieved target-range Hb values within 5 weeks after iron sucrose therapy without an increase in epoetin dose (physician-adjusted doses were permitted)
There were no serious adverse drug reactions recorded, no episodes of anaphylaxis, and no patient withdrawals or drug discontinuation caused by drug-related adverse events.2
Common adverse events associated with Venofer2
Adverse Effects
Number of Patients (n=77)
Diarrhea and abdominal pain
1 / 1.3 (%)
Diarrhea and nausea
1 / 1.3 (%)
Constipation
1 / 1.3 (%)
Transient minty taste
1 / 1.3 (%)
Intradialytic blood pressure (BP) changes with iron sucrose were similar to changes without iron sucrose2

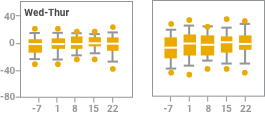
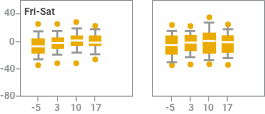
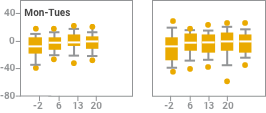
Effect of observation, iron sucrose treatment and dialysis day on systolic blood pressure change at intervals of 15 and 60 minutes after starting dialysis (observation; days -7, -5, and -2) or starting iron sucrose administration (treatment; days 1 to 22). Results are reported in box plots according to the days of the week in which dialysis sessions occurred. The centerline of each box represents the 50th percentile (median); the upper and lower extent of each box, the 75th and 25th percentiles, respectively; the capped error bars, the 90th and 10th percentiles; and the closed circles, the 95th and 5th percentiles. Intradialytic blood pressure changes after the administration of iron sucrose were not different from intradialytic changes seen without iron sucrose treatment.
NOTE: Blood pressure monitoring was part of the safety analysis of this study, not an outcome of the study. As part of the safety analysis, investigators reported intradialytic hypotension as an adverse event without reference to specific blood pressures or pressure changes.
Venofer demonstrated a positive effect on all the indices associated with anemia.2
Venofer administration does not require a test dose
Venofer administered as 1000 mg in 10 divided doses by IV push without a prior test dose for the treatment of iron deficiency in patients with dialysis-associated anemia.1
REFERENCE
1. Venofer® [package insert]. Shirley, NY: American Regent, Inc.; 2019.
STUDY REFERENCES
1. Venofer® [package insert]. Shirley, NY: American Regent, Inc.; 9/2020.
2. Charytan C, Levin N, Al-Saloum M, et al. Efficacy and safety of iron sucrose for iron deficiency in patients with dialysis-associated anemia: North American clinical trial. Am J Kidney Dis. 2001;37(2):300-307.

