Experience
Venofer® SELECTED CLINICAL STUDIES
Back to clinical studiesThis study led to the FDA approval of Venofer. Always refer to the approved Full Prescribing Information and Important Safety Information below.
Peritoneal-dialysis-dependent chronic kidney disease (PDD-CKD) patients with erythropoietin plus Venofer versus erythropoietin alone.1
PDD-CKD Venofer/Erythropoietin Patients Showed Greater Mean Change from Baseline2
Venofer is an effective adjunct to erythropoiesis-stimulating agents (ESA) therapy in PDD-CKD patients and is administered safely as 300 mg over 1.5 h or 400 mg over 2.5 h (a total dose of 1000 mg). Patients on ESA and Venofer showed increases in hemoglobin (Hb) and ferritin levels as high as 1.3 g/dL changes in Hb (versus 0.6 g/dL changes for ESA alone), while requiring fewer interventional treatments over 29 days (1 of 66 [1.3%] versus 5 of 30 [16.7%]).
Study Design/Methods1,2
This was an open-label, phase 3, randomized, multicenter trial. Anemic PDD-CKD patients on ESAs and required iron supplementation.
Study E was a randomized, open-label, multicenter study comparing patients with PDD-CKD receiving an erythropoietin and intravenous iron to patients with PDD-CKD receiving an erythropoietin alone without iron supplementation. Patients with PDD-CKD, stable erythropoietin for 8 weeks, hemoglobin of ≥ 8.5 g/dL to ≤ 11.5 g/dL, transferrin saturation (TSAT) ≤ 25%, or ferritin ≤ 500 ng/mL were randomized to receive either no iron or Venofer (300 mg in 250 mL 0.9% NaCl over 1.5 hours on day 1 and 15 and 400 mg in 250 mL 0.9% NaCl over 2.5 hours on day 29). The mean age of the 75 treated patients in the Venofer/erythropoietin group was 51.9 years (range 21 to 81 years) vs 52.8 years (range 23 to 77 years) for 46 patients in the erythropoietin-alone group. The principal analysis of the primary end point was the unstratified comparison of the peak Hb increase above baseline between the 2 study arms: group A (ESA + IV iron) and group B (ESA alone).
Secondary end points included the observed increase in TSAT and ferritin after treatment and the time to anemia intervention
Patients on ESA and Venofer had a longer time period without anemia intervention versus ESA alone2

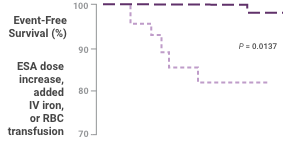
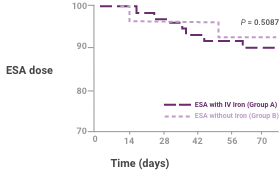
Effect of treatment on percentage of patients who remained without anemia intervention, including ESA dose increase, added intravenous iron, or transfusion for low hemoglobin (Hb) during the trial (top) or ESA dose decrease for elevated Hb (bottom). Results are shown for ITT patients. P-values are based on the Cox proportional hazard model with effects for treatment and baseline Hb.
Patients with ESA plus Venofer showed a higher increase in hemoglobin compared to erythropoietin alone2
Patients receiving ESA plus Venofer showed a higher peak Hb increase, the primary end point, than did those receiving erythropoietin alone (1.3 versus 0.6; difference 0.7 [95% confidence interval 0.3 to 1.2]; P = 0.0028).
A greater proportion of patients receiving ESA plus Venofer showed an increase in Hb ≥1.0 g/dL compared to patients receiving erythropoietin alone (59.1% versus 33.3%; P = 0.0273).

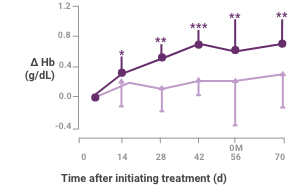
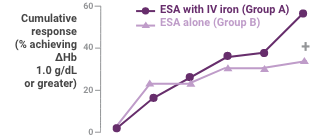
Hb response in anemic peritoneal dialysis patients after intravenous iron compared with no iron therapy. Proportion of patients who achieved a change in Hb ≥1.0 g/dL (top) and actual change in Hb (bottom; mean ± 95% confidence interval [CI] of mean). Between treatment groups, significant differences were present for cumulative response by day 71 (+P < 0.05). Within each group, significant differences in ΔHb from baseline are shown (*P < 0.05, **P < 0.001, ***P < 0.0001).
Between-group differences were significant for change in ferritin and TSAT at all time periods2

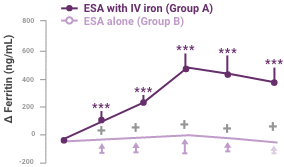
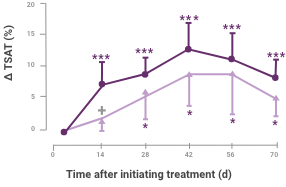
Change in iron status indicators in patients after IV iron compared with oral iron therapy (mean ± 95% CI of mean).
Between-group differences were significant for Δ ferritin at all time periods (P < 0.0001) and Δ transferrin saturation (ΔTSAT) at day 14 (P = 0.0314). Within each group, significant differences from baseline are shown (*P < 0.05, ***P < 0.001).
There were no serious adverse drug Events (ADE)2
ADE in group A safety-evaluable patients*
Adverse Side Effects
Group A (ESA + IV Iron; n = 75)
Gastrointestinal
5 (6.7%)
Constipation
1 (1.3%)
Diarrhea
2 (2.7%)
Nausea/vomiting
2 (2.7%)
Abdominal pain
1 (1.3%)
Pruritus and swelling
1 (1.3%)
Pruritus
1 (1.3%)
Discoloration at injection site
1 (1.3%)
Increased peripheral eosinophil count
1 (1.3%)
*Data are number of patients (% column total). All events were judged nonserious. One patient reported 2 gastrointestinal adverse drug events.
Three patients in group A discontinued as a result of adverse events; of these, one experienced an adverse event that was considered study drug-related. This patient experienced swelling of the feet and pruritus 30 to 45 min after completing her first 300-mg iron sucrose administration.
In this study, PDD-CKD patients who received ESA plus IV iron (group A) demonstrated the following outcomes compared to PDD-CKD patients receiving ESA alone (group B)2:
- Patients in group A showed a higher peak Hb increase, the primary end point, than did those in group B (1.3 vs 0.6; difference 0.7 [95% confidence interval 0.3 to 1.2]; P = 0.0028)
- When compared with patients who were assigned to group B, a greater portion of patients who were assigned to group A showed an increase in Hb ≥1.0 g/dL (59.1% vs 33.3% P = 0.0273)
- Serum ferritin increased significantly from baseline only in group A, and differences between groups were highly significant (P < 0.0001)
- Peak ferritin values attained were higher in group A than in group B (P < 0.0001) and correlated directly with baseline Hb (P = 0.0409) and baseline ferritin (P = 0.0004)
- Patients in group A experienced a higher rise in Hb, needed fewer anemia interventions and saw a greater decrease in net epoetin dose than patients who were given ESA therapy alone
STUDY REFERENCES
1. Venofer® [package insert]. Shirley, NY: American Regent, Inc.; 2019.
2. Singh H, Reed J, Noble S, et al. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1(3):475-482.

