Dosing & Administration
An established and effective treatment for chronic kidney disease (CKD) patients experiencing iron deficiency anemia (IDA).
*For the 25 years since FDA approval, Venofer® has been consistently available to the millions of patients in the US who need it. From June 2024 to November 2024 the product was on allocation but shipping weekly.


Artist rendering
†Venofer treatment may be repeated if IDA reoccurs.
Below are Venofer® (iron sucrose) injection, USP dosing and administration instructions for adult and pediatric patients over two years of age. For complete dosing information, always refer to the Full Prescribing Information.
Adult CKD Patients1
The usual adult total treatment course of Venofer is 1000 mg. Venofer treatment may be repeated if iron deficiency reoccurs.
|
IV Push |
100 mg over
|
|
|---|---|---|
|
200 mg over |
|
|
Infusion Diluted with 0.9% Sodium Chloride Injection at concentrations of 1 to 2 mg/mL |
100 mg in a maximum of 100 mL over at least 15 min |
|
|---|---|---|
|
200 mg in a maximum of 100 mL over 15 min |
|
|
|
2 infusions each of 300 mg in a maximum of 250 mL over 1.5 hrs followed by 1 dose of 400 mg in a maximum of 250 mL over 2.5 hrs |
|
‡Administer early during the dialysis session.
Pediatric CKD Patients1
Venofer treatment may be repeated if necessary. The dosing for iron replacement treatment in pediatric patients with hemodialysis-dependent chronic kidney disease (HDD-CKD), non-dialysis-dependent chronic kidney disease (NDD-CKD), or peritoneal-dialysis-dependent chronic kidney disease (PDD-CKD) has not been established.
|
Pediatric Patients (2 years of age or older) with HDD-CKD |
For iron maintenance treatment, administer Venofer
Do not dilute to concentrations below 1 mg/mL |
|---|
|
Pediatric Patients (2 years of age or older) with NDD-CKD or PDD-CKD who are on erythropoietin therapy for iron maintenance treatment |
For iron maintenance treatment, administer Venofer
Do not dilute to concentrations below 1 mg/mL |
|---|
Venofer has not been studied in patients younger than 2 years old.
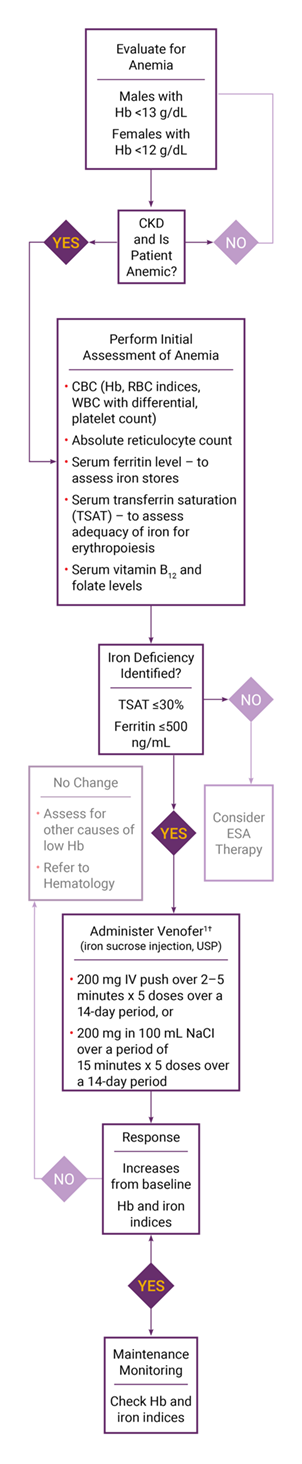

§ Adapted from the KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Official Journal of the International Society of Nephrology. Kidney Int. 2012;2(4):288-335.
‖ For adult CKD patients on ESA therapy who are not receiving iron supplementation, the guideline suggests a trial of IV iron (or in NDD-CKD patients, alternatively, a 1- to 3-month trial of oral iron therapy) if:
- An increase in Hb concentration or a decrease in ESA dose is desired and
- TSAT is <30% and ferritin is (≤500 µg/L)
CKD=chronic kidney disease; ESA=erythropoietin-stimulating agent; Hb=hemoglobin; RBC=red blood cell; WBC=white blood cell.
REFERENCE
1. Venofer (iron sucrose injection, USP) Package Insert. American Regent, Inc.

