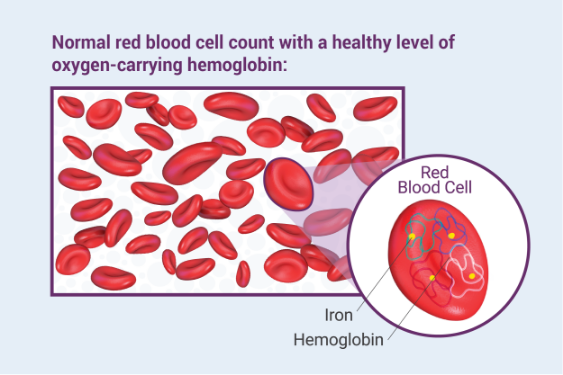
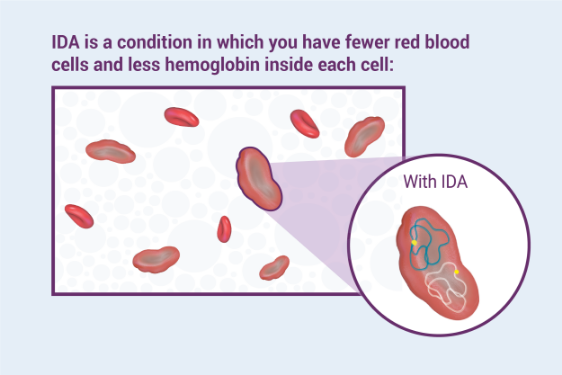
What are the possible side effects of Venofer?
- Serious allergic reactions: Some allergic reactions may be life-threatening, including shock, low blood pressure, and loss of consciousness. Other serious allergic reactions include itching, hives, swelling, wheezing, difficulty breathing, fainting, and/or other allergy symptoms, some of which have been life threatening and fatal, have happened in patients receiving Venofer.
- Low blood pressure: Venofer may cause significant decreases in blood pressure. Your healthcare provider will watch you closely for at least 30 minutes after each dose.
- Iron overload: Patients receiving Venofer require periodic blood tests to monitor the amount of iron in the blood.
Pregnancy:
Severe adverse reactions may occur in pregnant women including low blood pressure and shock, which may cause a dangerously slow heartbeat in the fetus, especially during the second and third trimester.
Most common side effects of Venofer:
- In adult patients, the most common side effects include diarrhea, upset stomach, throwing up, headache, dizziness, low blood pressure, itching, pain in the arms or legs, joint pain, back pain, muscle cramping, injection site reactions, chest pain, and swelling in the arms or legs.
- In children, the most common side effects are headache, respiratory virus, swelling of the stomach lining, throwing up, fever, dizziness, coughing, upset stomach, blood clots, and low or high blood pressure.
These are not all the side effects that may occur. Contact your healthcare provider if you have any questions or if you have symptoms that occur during or after you receive Venofer such as rash, itching, dizziness, light-headedness, swelling, or breathing problems.
The information provided is not intended to replace your healthcare provider’s medical advice. For additional information, contact your healthcare provider.
You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
REF-1405 6/2022